PeptideSynTM Peptide Synthesis Technology
 |
LifeTein routinely uses our proprietary technology to produce peptides >100 aa. The longest peptide we have made to date is 169aa. |
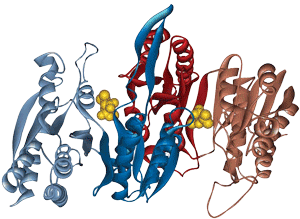
Catalytic active sites and most receptor binding sites for small ligands are found at domain and subunit interfaces. Long peptides are frequently synthesized to study the protein-receptor binding. Two active sites are indicated by the presence of the bound ligands (yellow). Both sites are at an interface between the two subunits of the protein.
Introducing Peptide Oligonucleotide Conjugate. Click here for more details.
Conventional SPPS typically requires a long coupling time of ≥1 hour, extended deprotection times, multiple washing steps, and Kaiser tests to ensure complete acylation. This time-consuming long synthesis process can be particularly troublesome when synthesizing complex peptides such as beta-amyloid or other large enzymes. The C-terminal segment of beta-amyloid is highly hydrophobic and is subject to subsequent on-resin aggregation. The rate of amino acid acylation is heavily dependent on the properties used for coupling. Therefore, the ability to assemble quality peptides faster is highly desirable.
LifeTein developed a platform that incorporates amino acid residues in a very short period of time. We are able to assemble multiple peptide fragments using native chemical ligation. The key process is to oxidize the C-terminal hydrazides into azide, followed by the addition of a thiol to generate thioesters for native chemical ligation.
LifeTein's PeptideSyn technology is a very practical platform for the synthesis of long peptides for use in research and for pharmaceutical purposes. PeptideSynTM technology has been used for the in-house testing of multiple selected biologically active peptides and enzymes. These include long and short peptides, as well as peptides containing D-amino acids or pseudoproline dipeptides. The processes have also used various coupling reagents such as HBTU, HATU, HCTU, ByBroP, BOP, PyBOP, and TBTU. PeptideSynTM technology allows us the flexibility to select affordable, highly efficient coupling reagents for rapid SPPS.
One study that used PeptideSynTM technology found that the synthesis of human beta-amyloid (1-42) (H-DAEFRHDSGYEVHHQKLVFFAEDVGSNKGAIIGLMVGGVVIA-OH) using HCTU as an activator required a total synthesis time of only 15 hours, and produced peptides of similar purity to those generated using long synthesis. The ability of Wang-ChemMatrix, HMPB-ChemMatrix, and Wang-PSLL to affect the efficiency of different synthesis procedures was compared. The C-terminal pentapeptide was incorporated using stepwise synthesis techniques. The coupling yields for the peptide segment were 90–95%. After treatment with hydrogen fluoride followed by purification, synthetic human beta-amyloid (1–42) peptide was obtained with an overall yield of 75–88%.
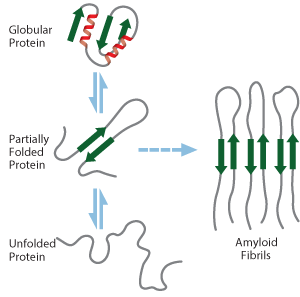
A possible mechanism for the formation of amyloid fibrils by a globular protein
LifeTein's PeptideSyn technology uses the appropriate coupling and deprotectant reagents linked to the correct resin to provide our customers with a reduced total peptide synthesis time and increased quality.
Case Study: A client requested a very hydrophobic 68-amino-acid peptide at 85% purity with a FITC modification at the N-terminus. The peptide was synthesized successfully in 2 weeks.
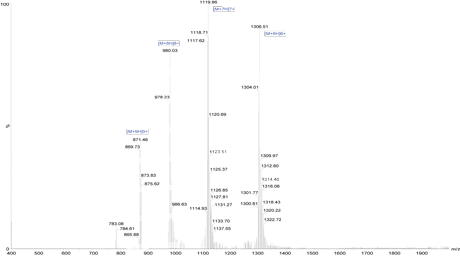
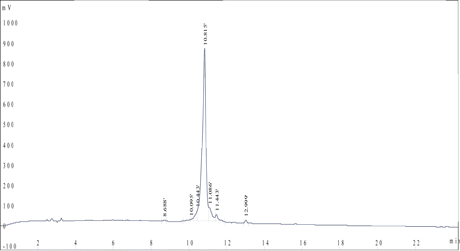
HPLC Results
Mass Spectrometry Results