Peptide Modifications: KLH, BSA, OVA Conjugates
Peptide-protein conjugates are used to produce antibodies against peptides. Because peptides alone are generally too small to elicit a sufficient immune response, carrier proteins that contain several epitopes are used to stimulate T-helper cells, which subsequently facilitate the induction of the B-cell response. Multiple antigenic peptides (MAPs) are an alternative approach that can be used to raise anti-peptide antibodies. This approach has several advantages over the traditional methodologies including eliminating the conjugation step, which can be time consuming and also result in random product mixtures.
The immune system reacts to the entire peptide-protein conjugate; therefore, some antibodies will always be raised against the peptide, the linker, and the carrier protein. As such, it is important to use a conjugate during immunization that differs from any that might be used in the final assays. For example, KLH conjugates should be used to immunize for antibodies when BSA will be used in the end-point assays. The most commonly used carrier proteins are as follows:
- KLH (keyhole limpet hemocyanin) is a copper-containing protein that is found in arthropods and mollusca. Therefore, it is an ideal carrier to use in mammalian hosts such as rabbits and mice. It is isolated from Megathura crenulata, and has a MW that ranges from 4.5 × 105 to 1.3 × 107 Da. KLH is the carrier that is used most commonly because it has a higher immunogenicity than does BSA. However, its solubility in water is limited because of its size and structure, which results in a cloudy appearance. The turbidity does not affect immunogenicity, and the resulting solution can be used for successful immunizations.
- BSA (bovine serum albumin) is a plasma protein in cattle that is one of the most stable and soluble albumins available. It has a MW of 67 × 103 Da, and includes 59 lysines. Of these, ~30–35 are accessible for use in linker conjugation, and so BSA is a popular carrier protein for weakly antigenic compounds. BSA is more water-soluble that KLH because it is smaller; therefore, it is used more commonly in immunoassays. However, because BSA is commonly used to block nonspecific binding sites in antibody-based assays BSA conjugates should not be used for immunization if the end-point assay system uses BSA. This is because if antisera against peptide-BSA conjugates are used in these assays, false positives are common because the sera used also contain antibodies against BSA.
- OVA (ovalbumin) is a protein isolated from hen egg whites, that has a MW of 45 × 103 Da. It is often used as a second carrier protein to confirm that antibodies are specific for the peptide rather than the carrier protein (e.g. BSA).
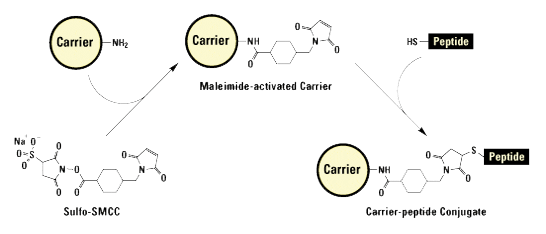
Thiol group modifications (via a Cys side chain) are used for KLH, BSA, or OVA conjugation.
All modifications that carry thiol-reactive functional groups can be used for conjugation. The most commonly used groups are as follows:
- Iodoacetamides
- Maleimides
- Alkyl halides
Determining the Peptide-to-Protein Ratio in the Conjugate
- Analyze the amino acid content of both the conjugate and native carrier protein
- Select one amino acid that is present in the carrier, but not in the peptide.
- Select an additional residue that is present in both the peptide and carrier protein.
- Calculate the ratio of these two amino acids in both the conjugate and native carrier.
- Determine the difference between these ratios, which reveals how many copies of the peptide are conjugated to the carrier protein. The example below shows that three copies of the peptide were conjugated to the carrier
|
Peptide |
Carrier Protein |
Conjugate |
| Ala |
1 |
10 |
13 |
| Val |
0 |
5 |
5 |
Estimating the peptide concentrations and coupling efficiency
Please visit this page at Sigma for technical details.
Conjugation Protocols
Conjugation is used to couple the antigenic peptide to either BSA or KLH. Because of the differences in the molecular weight of BSA and KLH, different calculations are used to quantify the amount of peptide that needs to be conjugated:
|
KLH |
BSA |
| Molecular Weight |
5 x 106 (average) |
67,000 |
| 5 mg of Protein |
1 nmol |
74.6 nmol |
For a 40-fold excess of peptide for KLH, the following formula is used:
(40 x 1 nmol = 40 nmol) x molecular weight of peptide = mg of peptide needed
For example, if 5 mg of KLH is used and the MW of the antigenic peptide is 2435, then a 40-fold excess = 98 µg of peptide. A 1-mg/mL solution of peptide is prepared in PBS, and 98 µL is used.
For a 40-fold excess of peptide for BSA conjugation, the formula would be:
(40 x 74.6 nmol = 3.0 µmol) x peptide molecular weight = mg of peptide needed.
For example, if 5 mg of BSA is used and the MW of the antigenic peptide is 2435, then a 40-fold excess = 7.3 mg peptide. A solution of 10 mg peptide in 100 µL of PBS is prepared, and 73 µL is used.
Conjugation using cysteine residues
- This type of conjugation requires either a N- or C-terminal cysteine.
- A total of 3 mg m-maleimidobenzoyl-N-hydroxysuccinimide ester (MBS) is dissolved in 200 ml dimethyl formamide (DMF)..
- Next, 70 ml MBS/DMF is added to a KLH solution consisting of 5 mg KLH in 0.5 ml 10 mM phosphate buffer, pH 7.0, and it is stirred gently at room temperature for 30 min.
- The free crosslinker is removed using a Sephadex G25 size exclusion column. First, the column is equilibrated in 50 mM phosphate buffer (pH 6.0). The KLH reaction mixture is then loaded, and eluted using 50 mM phosphate buffer (pH 6.0).
- A total of 5 mg synthetic peptide is dissolved in PBS or 6 M guanidine-HCl/10 mM phosphate buffer, pH 7.0, and is then added to the MB/KLH conjugate.
- The pH is adjusted to 7.3 using 0.1 M HCl or 0.1 M NaOH, and the mixture is stirred for 3 h at room temperature.
- The unbound peptide is removed using a Sephadex G25 column. The column is first equilibrated using ddH2O. The KLH reaction mixture is then loaded, and finally eluted using ddH2O.
- The conjugate is concentrated.
Please click here to get a peptide synthesis service quote now!
