CPPs Peptide Synthesis Modifications:
CPP-based cellular delivery vectors have enabled the introduction of a non-covalent strategy for associating ONs with carriers by simple mixing. However, the peptide sequence needs to be tuned carefully to that of both the cargo and application. For example, a stearylated poly-arginine peptide: Stearyl-R8: st-RRRRRRRR-NH2 was effective with a larger plasmid. In contrast, larger plasmids were ineffective for the cellular delivery of a splice-correcting oligonucleotide.

Method for simple mixing:
Example: To achieve MPG-mediated gene delivery, complexes of the peptide carrier and DNA, 500 ml of either DMEM or was mixed with 100 ng of DNA complexed with MPG at a charge ratio of 5:1. The samples were incubated at 37°C for 30 min, and then added to cells at 60% confluence.
Click this review paper from Nature to learn more about different peptide-mediated delivery systems.
Many novel CPPs have been used to effectively transfect a variety of cell types to elicit specific biological responses. For example, they were used to challenge primary and suspension cells without exerting cytotoxic effect. In addition, CPP-mediated ONs could inhibit tumor growth effectively in tumor-bearing mice in vivo, without triggering the immune response. Therefore, CPP–ON complexes have significant therapeutic potential.
Table: Application and effects of CPP-mediated siRNA delivery using noncovalent strategies
CPP |
Cells/Model |
Effect |
Reference |
MPG
ac-GALFLGFLGAAGSTMGAWSQPKKKRKV-cys
The cysteamide stabilises the DNA-carrier complexes. |
HeLa and Cos-7
HS68, NIH-3T3 |
Cellular uptake of single- and double-stranded ONs
50 nmol/l siRNA→85% and 78% luciferase
downregulation |
Simeoni et al. |
Stearyl-R8
st-RRRRRRRR-NH2
Order this peptide now!
FITC-Stearyl-R8 Control peptide
Polyarginine peptides have been efficiently used for siRNA delivery into cells.
Stearyl-R8 can lead nucleic acids through cell membranes. There is no need of covalently attachment between the nucleic acid and the multiple arginine peptide. |
EGFP-expressing
hippocampal neurons |
16 nmol/l siRNA→>50% reduction in EGFP activity |
Tönges et al. |
Tat-DRBD
GRKKRRQRRRPQ-DRBD |
H1299, HUVEC, Jurkat T,
hESC |
>90% reduction in GAPDH mRNA level |
Eguchi et al. |
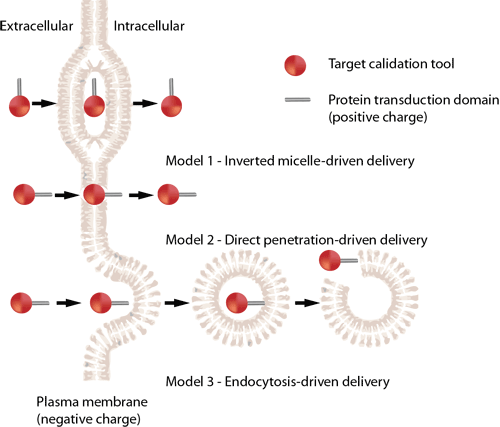
There are three proposed routes of CPP entry:
- Model 1: The inverted micelle model.
- Model 2: The direct penetration (pore formation) mechanism.
- Model 3: An endocytic mechanism of uptake.
Source: Cell-penetrating peptides and their therapeutic applications, Victoria Sebbage, BioscienceHorizons, Volume 2, Number 1, March 2009.
Arg or Lys-rich CPPs have been used widely for gene targeting. The stearly-RRRRRRRR-amide can be spaced using aminohexanoyl (Ahx) to enhance hydrophobicity and proteolytic resistance in serum. Adding a fatty acid to the Argnine rich peptides will enhance delivery of the peptide nucleic acid conjugate.
Click this review paper from Nature to learn more about the strategies for peptide-mediated delivery.
Since the discovery of Tat, the number of known peptides with cell-penetrating capabilities has grown. The following table shows some examples of currently known CPPs, together with their origins and sequences.
Name |
Origin |
Sequence |
Tat (48-60) |
HIV-1 protein |
GRKKRRQRRRPPQQ or GRKKRRQRRRPQ |
Oligoarginine |
Tat derivative |
Rn |
Transportan |
Galanin-mastoparan |
GWTLNSAGYLLGKINLKALAALAKKIL |
P-beta MPG peptides |
gp41-SV40 |
GALFLGFLGAAGSTMGAWSQPKKKRKV |
Pep-1 |
Trp-rich motif-SV40 |
KETWWETWWTEWSQPKKKRRV |
STR-R8 |
Tat derivative |
Stearly-RRRRRRRR-amide
(Stearyl = CH3(CH2)16CO-)
Order this peptide now!
FITC-Stearyl-R8 Control peptide
|
HIV TAT cell-penetrating peptides
CPPs, including the HIV TAT peptides, can enter cells via internalization pathways such as direct translocation and endocytosis. HIV TAT peptide or poly-arginine-containing peptides could be designed and utilized in drug delivery systems. However it is unclear how CPPs achieve efficient cellular molecular transportation.
How does HIV TAT peptide facilitate direct translocation and endocytosis? Experiments performed in the laboratory of Gerard Wong’s lab demonstrated that HIV TAT peptides could interact with several intracellular components, including the cell membrane, cell surface receptors, and the actin cytoskeleton. These interactions result in various translocation pathways under different conditions.

Interestingly, the same sequence within TAT peptide can interact with different cellular components, including the plasma membrane, or the actin cytoskeleton to activate different translocation pathways under a variety of conditions.
The entry mechanisms of cell penetrating peptide depend on their amino acid sequence. Adding one hydrophobic amino acid to a highly hydrophilic cell penetrating peptide sequence could significantly change the penetration mechanism. For example, the polyarginine RRRRRRRR could induce the formation of a cell membrane pore. The arginine creates positive and negative curvatures at the same time. However lysine creates only negative curvature in a single direction. So a peptide with arginine residues and lysine or hydrophobic amino acids could function differently.
TAT peptide can mediate endocytosis both with and without receptors. CPPs use less hydrophobic amino acids to create curvature in the membrane. High levels of hydrophobicity generate negative Gaussian curvature.